Chemistry Resources
The grade ten chemistry unit concentrates on chemical properties and reactions. These resources are grouped by the chapters in the Nelson Science 10 textbook, because that arrangement makes sense to me (a non-chemist).
Copyright on all materials on this site is retained by the authors. You are granted a limited license to reproduce these resources for classroom use, provided the copyright notices are not removed. Charging a fee for these resources, or distributing them in any way outside your classroom, is prohibited.
General Chemistry
The resourcees here either relate to the entire unit or address overall expectations.
Instructional Resources
Biography of a Chemist
We are supposed to be teaching a bit of the history of science (expectation A2.2) as well as facts and theories, and yet often we lose track of the human nature of science and scientists. This short writing assignment has students research one chemist and write a five-paragraph story about them.
I use this assignment to reinforce both research skills and writing skills.
I use this assignment to reinforce both research skills and writing skills.
Chemist Cards
I created these to end arguments over who got to write about which chemist. I print them out and randomly distribute them in class. Students can trade them if they like, but must write about the chemist they end up with. Has all the chemists in the Biography of a Chemist assignment, plus a few extra to make up 40 chemists.
Oddly, some of my students wanted to use them as trading cards.I'm thinking of making up a set for every unit, maybe with short biographic blurbs, and handing them out as rewards!
Oddly, some of my students wanted to use them as trading cards.I'm thinking of making up a set for every unit, maybe with short biographic blurbs, and handing them out as rewards!
Chemistry: A Volatile History
Chemistry: A Volatile History is a 2010 BBC documentary on the history of chemistry presented by Jim Al-Khalili. It was nominated for the 2010 British Academy Television Awards in the category Specialist Factual.
Zombie College
A lab safety website from NC Community Colleges, featuring a short film, music video, video game, and lesson plans.
Whether you're a chemist, researcher, or a student in a freshman biology course, the laboratory is a potentially fatal workplace. Lab safety is more than just memorizing a list of "Dos and Don'ts"...working safely is a state of mind. Our challenge was to design a blended learning program to serve as a catalyst to get learners thinking about and discussing the topic of lab safety. The ultimate goal is developing "a safety state of mind" which is demonstrated by behavioral change.
With Zombie College, lab safety training comes to life!
Whether you're a chemist, researcher, or a student in a freshman biology course, the laboratory is a potentially fatal workplace. Lab safety is more than just memorizing a list of "Dos and Don'ts"...working safely is a state of mind. Our challenge was to design a blended learning program to serve as a catalyst to get learners thinking about and discussing the topic of lab safety. The ultimate goal is developing "a safety state of mind" which is demonstrated by behavioral change.
With Zombie College, lab safety training comes to life!
Updated October 24, 2013
Safety Poster
Safety is very important when doing labs, especially chemistry labs. I distribute the safety rules from the textbook among the class (that’s why there’s a space to write the rule on the sheet) and have students make a small poster. The best ones get displayed around the classroom as reminders.
Lab Safety Tableaux
Safety is the primary concern in any science lab. One way to remember safety rules is to deliberately break them — safely.
In this assignment students create tableaux showing themselves breaking lab safety rules, and take pictures of the tableaux. With each picture they provide a list of all the safety rules being broken in the tableau. (which shows that they understand what is dangerous).
Two slightly different versions of the assignment are included.
In this assignment students create tableaux showing themselves breaking lab safety rules, and take pictures of the tableaux. With each picture they provide a list of all the safety rules being broken in the tableau. (which shows that they understand what is dangerous).
Two slightly different versions of the assignment are included.
Hands-On Activities
Elements Matching Game
Cards for a game involving matching elements and symbols. Sized to use standard business card stock.
The cards can also be printed as flashcards, with the symbol on the front and the element on the back.
The cards can also be printed as flashcards, with the symbol on the front and the element on the back.
Element Bingo
A simple set of bingo cards for the first 20 elements, plus 11 others that we often use (like copper, silver, and iron).
More a review of grade 9 than grade 10 material, this can be handy for applied-level students.
Contains 200 bingo cards, element chits to draw from a hat, and a caller’s card to keep track of what you’ve called.
More a review of grade 9 than grade 10 material, this can be handy for applied-level students.
Contains 200 bingo cards, element chits to draw from a hat, and a caller’s card to keep track of what you’ve called.
Supplementary Resources
In Our Time: Alchemy
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and guests discuss the history of Alchemy, the ancient science of transformations. The most famous alchemical text is the Emerald Tablet, written around 500BC and attributed to the mythical Egyptian figure of Hermes Trismegistus. Among its twelve lines are the essential words — “as above, so below”. They capture the essence of alchemy, that the heavens mirror the earth and that all things correspond to one another. Alchemy was taken up by some of the most extraordinary people in our intellectual development, including Roger Bacon, Paracelsus, the father of chemistry, Robert Boyle, and, most famously, Isaac Newton, who wrote more about alchemy than he did about physics. It is now contended that it was Newton’s studies into alchemy which gave him the fundamental insight into the famous three laws of motion and gravity.
Melvyn Bragg and guests discuss the history of Alchemy, the ancient science of transformations. The most famous alchemical text is the Emerald Tablet, written around 500BC and attributed to the mythical Egyptian figure of Hermes Trismegistus. Among its twelve lines are the essential words — “as above, so below”. They capture the essence of alchemy, that the heavens mirror the earth and that all things correspond to one another. Alchemy was taken up by some of the most extraordinary people in our intellectual development, including Roger Bacon, Paracelsus, the father of chemistry, Robert Boyle, and, most famously, Isaac Newton, who wrote more about alchemy than he did about physics. It is now contended that it was Newton’s studies into alchemy which gave him the fundamental insight into the famous three laws of motion and gravity.
In Our Time: Chemical Elements
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and guests discuss the chemical elements. The aim and challenge in chemistry, according to the Encyclopaedia Britannica, is the understanding of the complex materials which constitute everything in existence since the Big Bang, when the whole universe emerged out of the two elements of hydrogen and helium.
Today we have the key to understanding these elements, the Periodic Table, which is a pattern embedded in nature and was miraculously discovered in a dream.
Melvyn Bragg and guests discuss the chemical elements. The aim and challenge in chemistry, according to the Encyclopaedia Britannica, is the understanding of the complex materials which constitute everything in existence since the Big Bang, when the whole universe emerged out of the two elements of hydrogen and helium.
Today we have the key to understanding these elements, the Periodic Table, which is a pattern embedded in nature and was miraculously discovered in a dream.
In Our Time: Oxygen
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg discusses the discovery of Oxygen by Joseph Priestley and Antoine Lavoisier.
For the British dissenting preacher, Joseph Priestley, and the French aristocrat, Antoine Lavoisier, Chemistry was full of possibilities and they pursued them for scientific and political ends. But they came to blows over oxygen because they both claimed to have discovered it, provoking a scientific controversy that rattled through the laboratories of France and England until well after their deaths. To understand their disagreement is to understand something about the nature of scientific discovery itself.
Melvyn Bragg discusses the discovery of Oxygen by Joseph Priestley and Antoine Lavoisier.
For the British dissenting preacher, Joseph Priestley, and the French aristocrat, Antoine Lavoisier, Chemistry was full of possibilities and they pursued them for scientific and political ends. But they came to blows over oxygen because they both claimed to have discovered it, provoking a scientific controversy that rattled through the laboratories of France and England until well after their deaths. To understand their disagreement is to understand something about the nature of scientific discovery itself.
In Our Time: Robert Boyle
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and his guests discuss the life and work of Robert Boyle, a pioneering scientist and a founder member of the Royal Society. Born in Ireland in 1627, Boyle was one of the first natural philosophers to conduct rigorous experiments, laid the foundations of modern chemistry and derived Boyle's Law, describing the physical properties of gases. In addition to his experimental work he left a substantial body of writings about philosophy and religion; his piety was one of the most important factors in his intellectual activities, prompting a celebrated dispute with his contemporary Thomas Hobbes.
Melvyn Bragg and his guests discuss the life and work of Robert Boyle, a pioneering scientist and a founder member of the Royal Society. Born in Ireland in 1627, Boyle was one of the first natural philosophers to conduct rigorous experiments, laid the foundations of modern chemistry and derived Boyle's Law, describing the physical properties of gases. In addition to his experimental work he left a substantial body of writings about philosophy and religion; his piety was one of the most important factors in his intellectual activities, prompting a celebrated dispute with his contemporary Thomas Hobbes.
Elemental Sudoku
I got these from the Royal Society of Chemistry. If you have students who can't put away their sudoku puzzles, try them on these! Answers are included.
Chemical Properties
These resources relate to chemical properties and chemical bonding.
Instructional Resources
Clumps of Atoms
A six-page look at elements and molecules, written by Dr. David Morgan-Mar and provided by him under a Creative Commons license.
We’ve talked about elements and what they are made of, namely atoms. The ancient Greeks thought there were just four elements, and that everything else was made of combinations of those. The basic idea was right, they just got the actual elements wrong. We know now that neither air, fire, earth, nor water are elements. The question then comes to mind: What are they?
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to chemistry.
We’ve talked about elements and what they are made of, namely atoms. The ancient Greeks thought there were just four elements, and that everything else was made of combinations of those. The basic idea was right, they just got the actual elements wrong. We know now that neither air, fire, earth, nor water are elements. The question then comes to mind: What are they?
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to chemistry.
Organising the Elements
A six-page history of the periodic table, written by Dr. David Morgan-Mar and provided by him under a Creative Commons license.
Stuff is made of atoms, tiny particles that are too small to see. We can infer the existence of atoms from the chemical properties of matter, in particular how elements combine to form other substances. For many common compounds, the elements combine in specific fixed ratios, which can be understood as the combination of specific numbers of atoms of each element into molecules.
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to the periodic table.
Stuff is made of atoms, tiny particles that are too small to see. We can infer the existence of atoms from the chemical properties of matter, in particular how elements combine to form other substances. For many common compounds, the elements combine in specific fixed ratios, which can be understood as the combination of specific numbers of atoms of each element into molecules.
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to the periodic table.
Ionic Bonding Practice
I don't believe in overloading students with homework, but I do like to give them enough practice that they feel confident that they understand. This sixteen page workbook gives them lots of practice forming and naming ionic compounds. Answers are provided so they can check their understanding.
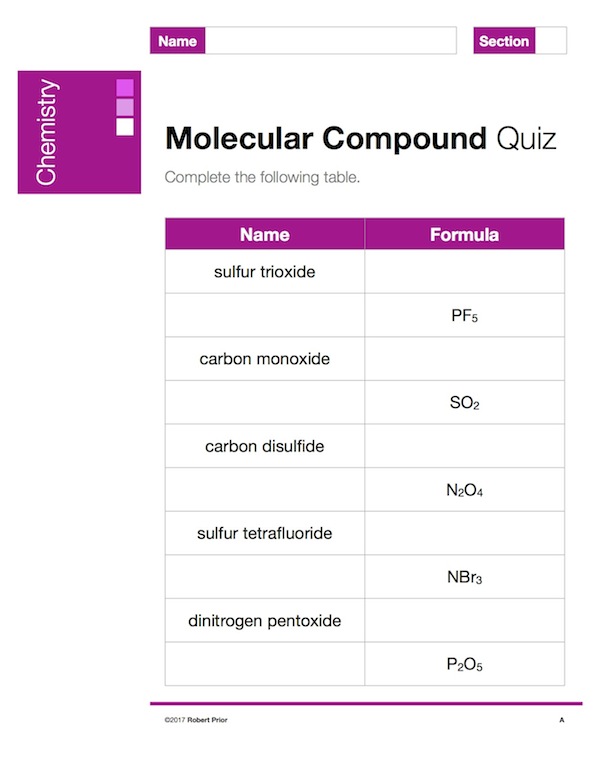
Ionic Compound Quizzes
I give a lot of quizzes for ionic bonding, because I want students to master it. Although marks at first are low, this doesn’t matter because I use “most recent most consistent”, so as long as students make progress (and almost all do) the slower learners aren’t penalized. This package contains ten quizzes, with answers.
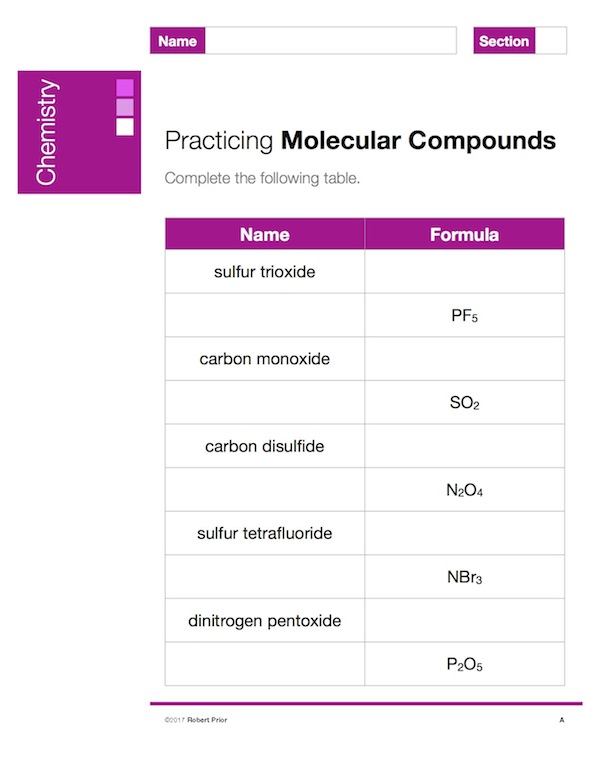
Molecular Compound Exercises
A set of five worksheets to practice naming molecular compounds from the formula, or writing the formula from the name. Answers are provided.
Carbon
A six-page tour of carbon and organic chemistry, written by Dr. David Morgan-Mar and provided by him under a Creative Commons license.
Take six protons, six neutrons, and six electrons and assemble them into an atom. You have produced an atom of carbon, one of the most common elements in the universe, and exceedingly easy to find on the surface of the Earth. Carbon is an extraordinary element, underlying much of our existence.
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to organic chemistry.
Take six protons, six neutrons, and six electrons and assemble them into an atom. You have produced an atom of carbon, one of the most common elements in the universe, and exceedingly easy to find on the surface of the Earth. Carbon is an extraordinary element, underlying much of our existence.
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to organic chemistry.
Hands-On Activities
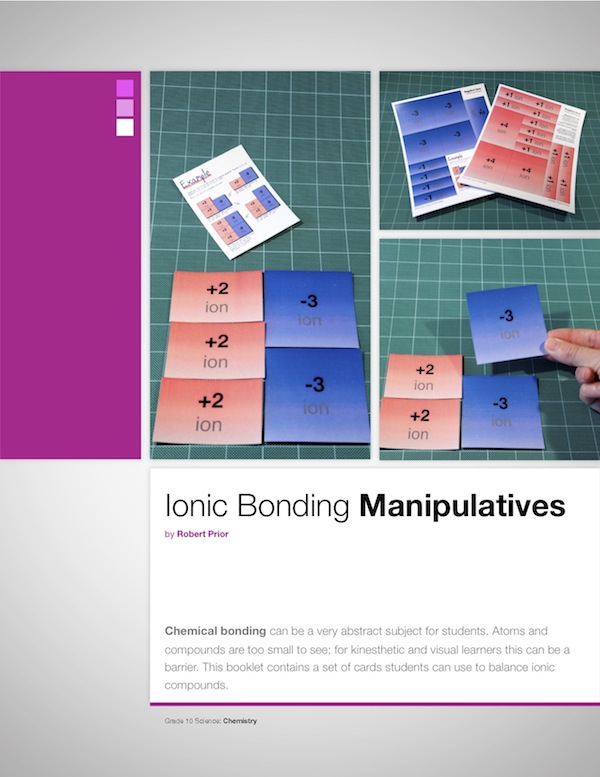
Ionic Bonding Manipulatives
Some students understand balancing ionic charges immediately; others get lost in the numbers and struggle. These manipulatives make balancing an ionic compound a visual procedure, rather than a mathematical one. I’ve found that if I allow students to use the manipulatives whenever they want, including quizzes and tests, they stop on their own once the balancing ‘clicks’ for them.
IoniCompounds Game
IoniCompounds is a fun way for students to practice forming ionic compounds. The unique ion cards make it easy to visually balance ionic compounds, while the special cards keep play exciting until the last turn.
This file contains rulebook, cards, and a folding storage box — everything needed to play IoniCompounds. The rulebook should be printed double-sided, folded, and stapled to make a small booklet. The cards can be printed on pre-punched business card stock, or printed on card stock and cut out. Alternate card backs make multiple classroom sets easy to sort out if your students get them mixed up.
This file contains rulebook, cards, and a folding storage box — everything needed to play IoniCompounds. The rulebook should be printed double-sided, folded, and stapled to make a small booklet. The cards can be printed on pre-punched business card stock, or printed on card stock and cut out. Alternate card backs make multiple classroom sets easy to sort out if your students get them mixed up.
ION: A Compound Building Game
ION: A Compound Building Game is a simple card drafting game where players select from a number of available ion cards and noble gas cards, with the objective of forming either neutrally charged compounds or sets of stable noble gases.
How to Play
Each player is initially dealt eight cards. They choose one card and pass the remaining to the player on their left, while they receive the same amount of cards from the player on their right (this is commonly referred to as “card drafting” or “pick and pass”).
Selected cards must be either (1) bonded to another ion or (2) set alone. Players only score points for neutrally balanced cards. So a positive charged Sodium (Na+) bonding with a negatively charge Chloride (Cl-), forming a neutral NaCl compound. would score points.
Points are scored at the end of each round and player may gain additional points for building specific compounds listed on the goal cards for that round. After three rounds the player with the most points wins!
The game comes with multiple expansions including a Transition Metals expansion, a Polyatomic Ion expansion, and a Radioactive Card expansion.
This game take a different approach to my own Ionicompounds game.
How to Play
Each player is initially dealt eight cards. They choose one card and pass the remaining to the player on their left, while they receive the same amount of cards from the player on their right (this is commonly referred to as “card drafting” or “pick and pass”).
Selected cards must be either (1) bonded to another ion or (2) set alone. Players only score points for neutrally balanced cards. So a positive charged Sodium (Na+) bonding with a negatively charge Chloride (Cl-), forming a neutral NaCl compound. would score points.
Points are scored at the end of each round and player may gain additional points for building specific compounds listed on the goal cards for that round. After three rounds the player with the most points wins!
The game comes with multiple expansions including a Transition Metals expansion, a Polyatomic Ion expansion, and a Radioactive Card expansion.
This game take a different approach to my own Ionicompounds game.
Rare Earth: Chemical Element Card Game
From cute little Hydrogen to heroic Meitnerium to mysterious Ununoctium, chemical element cartoon characters are bonding together, mixing-it-up and raiding each other’s Labs to capture Protons in this exciting card game.
With the Rare Earth Chemical Element Card Game, every child has fun learning the fundamentals of Chemistry, Great for Science classrooms and entertaining for kids and families, players win by combining chemical element cards.
This game is built around forming binary ionic compounds. Bonding a new compound gets you an energy card, which lets you mix an alloy. Forming a compound or allow also lets you steal a compound or allow from one of your opponents.
An expansion pack is available that adds cards to make a complete periodic table.
The rules include several variants, making it suitable for different grades. For the basic game you don't need to know any chemistry — it’s built into the rules. I would start with the simplest version (intended for children) and add complexity if the class likes the game.
Rare Earth is designed for 2-6 players, so the average classroom would need 5-6 sets. Fortunately, there’s a classroom price: six sets plus an expansion pack for the price of five, with free shipping.
LeapCloud is a Canadian company, which makes shipping a lot more reasonable than it is for many products (and free if you order two products at once).
With the Rare Earth Chemical Element Card Game, every child has fun learning the fundamentals of Chemistry, Great for Science classrooms and entertaining for kids and families, players win by combining chemical element cards.
This game is built around forming binary ionic compounds. Bonding a new compound gets you an energy card, which lets you mix an alloy. Forming a compound or allow also lets you steal a compound or allow from one of your opponents.
An expansion pack is available that adds cards to make a complete periodic table.
The rules include several variants, making it suitable for different grades. For the basic game you don't need to know any chemistry — it’s built into the rules. I would start with the simplest version (intended for children) and add complexity if the class likes the game.
Rare Earth is designed for 2-6 players, so the average classroom would need 5-6 sets. Fortunately, there’s a classroom price: six sets plus an expansion pack for the price of five, with free shipping.
LeapCloud is a Canadian company, which makes shipping a lot more reasonable than it is for many products (and free if you order two products at once).
Supplementary Resources
In Our Time: Carbon
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and guests discuss Carbon. It forms the basis of all organic life and has the amazing ability to bond with itself and a wide range of other elements, forming nearly 10 million known compounds. It is in the food we eat, the clothes we wear, the shampoo we use and the petrol that fuels our cars. Because carbon has the largest range of subtle bonding capabilities, 95% of everything that exists in the universe is made up of carbon atoms that are stuck together.
Melvyn Bragg and guests discuss Carbon. It forms the basis of all organic life and has the amazing ability to bond with itself and a wide range of other elements, forming nearly 10 million known compounds. It is in the food we eat, the clothes we wear, the shampoo we use and the petrol that fuels our cars. Because carbon has the largest range of subtle bonding capabilities, 95% of everything that exists in the universe is made up of carbon atoms that are stuck together.
In Our Time: Water
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and his guests discuss one of the simplest and most remarkable of all molecules: water. Water is among the most abundant substances on Earth, covering more than two-thirds of the planet. Consisting of just three atoms, the water molecule is superficially simple in its structure but extraordinary in its properties. It is a rare example of a substance that can be found on Earth in gaseous, liquid and solid forms, and thanks to its unique chemical behaviour is the basis of all known life. Scientists are still discovering new things about it, such as the fact that there are at least fifteen different forms of ice.
Melvyn Bragg and his guests discuss one of the simplest and most remarkable of all molecules: water. Water is among the most abundant substances on Earth, covering more than two-thirds of the planet. Consisting of just three atoms, the water molecule is superficially simple in its structure but extraordinary in its properties. It is a rare example of a substance that can be found on Earth in gaseous, liquid and solid forms, and thanks to its unique chemical behaviour is the basis of all known life. Scientists are still discovering new things about it, such as the fact that there are at least fifteen different forms of ice.
Chemical Reactions
These resources relate to chemical reactions.
Instructional Resources
Chemical Equation Practice
I don't believe in overloading students with homework, but I do like to give them enough practice that they feel confident that they understand. This 22 page workbook gives them lots of practice writing and balancing chemical equations. Answers are provided so they can check their understanding.
Chemical Equation Quizzes
I give a lot of quizzes for chemical equations, because I want students to master them. Although marks at first are low, this doesn't matter because I use "most recent most consistent", so as long as students make progress (and almost all do) the slower learners aren't penalized. This package contains ten quizzes, with answers.
Hands-On Activities
Model Cards for Balancing Reactions
Students often have a hard time balancing equations. They get lost in the numbers — subscripts, superscripts, how many atoms of each element? — and lose track of what they're trying to do.
This activity uses pictures of molecules to balance equations. it includes teacher’s notes, a student worksheet, ten pages of molecular model cards, template equations (for applied classes), and labels for bags to store the cards in. With most classes it can be completed in a shortened period.
This activity uses pictures of molecules to balance equations. it includes teacher’s notes, a student worksheet, ten pages of molecular model cards, template equations (for applied classes), and labels for bags to store the cards in. With most classes it can be completed in a shortened period.
Corrosion Activity
A simple activity based on the corrosion of iron. These are the student worksheets — I’ll add teachers’ notes when I have time to write them up.
In essence, show students the raw materials for a case of corrosion and get them to predict what will happen, and explain their prediction. Then show them the (previously prepared) corroded object and get them to write observations, explain their observations, and then apply what they have learned to daily life.
Two versions are included: one with a marking rubric, one with just a space for teacher comments.
In essence, show students the raw materials for a case of corrosion and get them to predict what will happen, and explain their prediction. Then show them the (previously prepared) corroded object and get them to write observations, explain their observations, and then apply what they have learned to daily life.
Two versions are included: one with a marking rubric, one with just a space for teacher comments.
Acids and Bases
These resources relate to acids and bases. Note that some aspects of the climate unit (such as ocean acidification) can also be addressed here.
Instructional Resources
Acids and Bases Lessons
A presentation of acids and bases, rendered as a QuickTime file for maximum portability. This isn't stand-alone, but intended as part of a lecture presentation. Click the mouse (or use the spacebar) to advance to the next build.
Acids and Bases
A six-page explanation of the pH scale, written by Dr. David Morgan-Mar and provided by him under a Creative Commons license.
It wasn’t until I got to university that I started to appreciate chemistry again. The reason, I think, is because that up until then chemistry seems to be more or less arbitrary rules that you just have to know. To understand why those rules exist, you have to dig deeper than high school science allows. You need to appreciate some of the intricacies of quantum mechanics, which dictates things like the numbers of electrons in the shells of atoms.
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to acids and bases.
It wasn’t until I got to university that I started to appreciate chemistry again. The reason, I think, is because that up until then chemistry seems to be more or less arbitrary rules that you just have to know. To understand why those rules exist, you have to dig deeper than high school science allows. You need to appreciate some of the intricacies of quantum mechanics, which dictates things like the numbers of electrons in the shells of atoms.
I'm field-testing a set of questions and directed reading exercises for this resource, and I'll include them with the download when they are ready (and announce the update). In the meantime, enjoy an interesting and well-written introduction to acids and bases.
Hands-On Activities
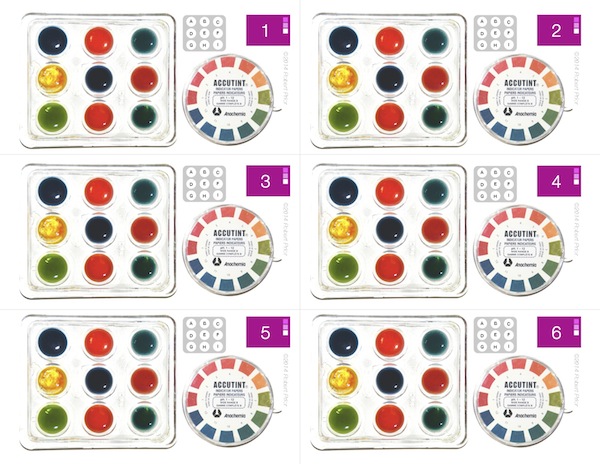
Red Cabbage Experiment
A simple experiment using red cabbage juice as a pH indicator. Two versions are included: one using distilled water, and one using tap water. (Tap water works quite well for this experiment.)
Nelson OSSLT Questions
The Nelson Science 10 textbook includes at least one reading assignment per chapter specifically geared to practicing for the Ontario Secondary School Literacy Test. These questions relate to those readings. If you don't use the Nelson Science 10 textbook, they will be of no use to you.































Teaching Science