Grade 12 Chemistry, University Preparation
This course enables students to deepen their understanding of chemistry through the study of organic chemistry, the structure and properties of matter, energy changes and rates of reaction, equilibrium in chemical systems, and electrochemistry. Students will further develop their problem-solving and investigation skills as they investigate chemical processes, and will refine their ability to communicate scientific information.
I've never taught this course, but here is material I think would be useful.
Copyright on all materials on this site is retained by the authors. You are granted a limited license to reproduce these resources for classroom use, provided the copyright notices are not removed. Charging a fee for these resources, or distributing them in any way outside your classroom, is prohibited.
General Chemistry
Word Puzzle Booklet
Science has a lot of specialized vocabulary. Some students like word puzzles, so I made a booklet of crossword puzzles and word search puzzles for them. The clues are the definitions from the Nelson Chemistry 12 textbook. Every chapter has both a crossword puzzle and a word search puzzle, and so does every unit. Answers are given at the back.
The booklet is designed to be printed double-sided, which puts the clues on the left page and the puzzle on the right page.
The booklet is designed to be printed double-sided, which puts the clues on the left page and the puzzle on the right page.
Organic Chemistry
Covalence: A Molecule Building Game
Covalence is a chemistry-themed cooperative game where players work together to accurately build a number of secret organic molecules. One player has knowledge of a set of Secret Molecules. All other players must deduce what these secret molecules are, based upon clues given to them. Players must cooperatively construct their molecules before the clues run out!
How to Play
One player takes on the role of the “Knower” while all other players then become the “Builders”. The Knower has access to a number of Secret Molecules and will give the Builders clues about these as they attempt to identify their structure. After setting up the Clue cards in front of her, the Knower will randomly designate 3 Secret Molecule cards for each Builder (4 Secret Molecules if there is only one Builder). Each Builder then takes a set of Element Tiles that bear a variety of elements and bonding patterns.
The Knower must study the Secret Molecules designated to each Builder and then give each Builder clue cards that relate to their specific Molecule!
Each Builder must interpret these Clues as they arrange and rearrange their individual Element Tiles, attempting to deduce the structure of their Secret Molecule.
Builders may request new Clues from the Knower as the game progresses with their limited supply of Clue Tokens.
When the Builders think that the molecular structures they have built match their Secret Molecules, they submit their structure to the Knower with a Guess Token.
If the Builders submit their molecular structures and are wrong, they have exhausted precious resources and are that much closer to losing the game.
If the Builders submit their molecular structures and are correct, they receive more Clue Tokens as a reward and may begin building their next Secret Molecule! If each Builder is able to correctly build both of their Secret Molecules, everyone wins!
You don’t need to know chemistry to play this game. I don’t teach organic chemistry, but I think this might be useful as a light-hearted review.
How to Play
One player takes on the role of the “Knower” while all other players then become the “Builders”. The Knower has access to a number of Secret Molecules and will give the Builders clues about these as they attempt to identify their structure. After setting up the Clue cards in front of her, the Knower will randomly designate 3 Secret Molecule cards for each Builder (4 Secret Molecules if there is only one Builder). Each Builder then takes a set of Element Tiles that bear a variety of elements and bonding patterns.
The Knower must study the Secret Molecules designated to each Builder and then give each Builder clue cards that relate to their specific Molecule!
Each Builder must interpret these Clues as they arrange and rearrange their individual Element Tiles, attempting to deduce the structure of their Secret Molecule.
Builders may request new Clues from the Knower as the game progresses with their limited supply of Clue Tokens.
When the Builders think that the molecular structures they have built match their Secret Molecules, they submit their structure to the Knower with a Guess Token.
If the Builders submit their molecular structures and are wrong, they have exhausted precious resources and are that much closer to losing the game.
If the Builders submit their molecular structures and are correct, they receive more Clue Tokens as a reward and may begin building their next Secret Molecule! If each Builder is able to correctly build both of their Secret Molecules, everyone wins!
You don’t need to know chemistry to play this game. I don’t teach organic chemistry, but I think this might be useful as a light-hearted review.
In Our Time: Free Radicals
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and guests discuss the properties of atoms or molecules with a single unpaired electron, which tend to be more reactive, keen to seize an electron to make it a pair. In the atmosphere, they are linked to reactions such as rusting. Free radicals came to prominence in the 1950s with the discovery that radiation poisoning operates through free radicals, as it splits water molecules and produces a very reactive hydroxyl radical which damages DNA and other molecules in the cell. There is also an argument that free radicals are a byproduct of normal respiration and over time they cause an accumulation of damage that is effectively the process of ageing. For all their negative associations, free radicals play an important role in signalling and are also linked with driving cell division, both cancer and normal cell division, even if they tend to become damaging when there are too many of them.
Melvyn Bragg and guests discuss the properties of atoms or molecules with a single unpaired electron, which tend to be more reactive, keen to seize an electron to make it a pair. In the atmosphere, they are linked to reactions such as rusting. Free radicals came to prominence in the 1950s with the discovery that radiation poisoning operates through free radicals, as it splits water molecules and produces a very reactive hydroxyl radical which damages DNA and other molecules in the cell. There is also an argument that free radicals are a byproduct of normal respiration and over time they cause an accumulation of damage that is effectively the process of ageing. For all their negative associations, free radicals play an important role in signalling and are also linked with driving cell division, both cancer and normal cell division, even if they tend to become damaging when there are too many of them.
In Our Time: Dorothy Hodgkin
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and guests discuss the work and ideas of Dorothy Crowfoot Hodgkin (1910-1994), awarded the Nobel Prize in Chemistry in 1964 for revealing the structures of vitamin B12 and penicillin and who later determined the structure of insulin. She was one of the pioneers of X-ray crystallography and described by a colleague as 'a crystallographers' crystallographer'. She remains the only British woman to have won a Nobel in science, yet rejected the idea that she was a role model for other women, or that her career was held back because she was a woman. She was also the first woman since Florence Nightingale to receive the Order of Merit, and was given the Lenin Peace Prize in recognition of her efforts to bring together scientists from the East and West in pursuit of nuclear disarmament.
Melvyn Bragg and guests discuss the work and ideas of Dorothy Crowfoot Hodgkin (1910-1994), awarded the Nobel Prize in Chemistry in 1964 for revealing the structures of vitamin B12 and penicillin and who later determined the structure of insulin. She was one of the pioneers of X-ray crystallography and described by a colleague as 'a crystallographers' crystallographer'. She remains the only British woman to have won a Nobel in science, yet rejected the idea that she was a role model for other women, or that her career was held back because she was a woman. She was also the first woman since Florence Nightingale to receive the Order of Merit, and was given the Lenin Peace Prize in recognition of her efforts to bring together scientists from the East and West in pursuit of nuclear disarmament.
Structure and Properties of Matter
In Our Time: The Science of Glass
In Our Time is a wonderful series on BBC Radio 4.
While glass items have been made for at least 5,000 years, scientists are yet to explain, conclusively, what happens when the substance it's made from moves from a molten state to its hard, transparent phase. It is said to be one of the great unsolved problems in physics. While apparently solid, the glass retains certain properties of a liquid. At times, ways of making glass have been highly confidential; in Venice in the Middle Ages, disclosure of manufacturing techniques was a capital offence. Despite the complexity and mystery of the science of glass, glass technology has continued to advance from sheet glass to crystal glass, optical glass and prisms, to float glasses, chemical glassware, fibre optics and metal glasses.
While glass items have been made for at least 5,000 years, scientists are yet to explain, conclusively, what happens when the substance it's made from moves from a molten state to its hard, transparent phase. It is said to be one of the great unsolved problems in physics. While apparently solid, the glass retains certain properties of a liquid. At times, ways of making glass have been highly confidential; in Venice in the Middle Ages, disclosure of manufacturing techniques was a capital offence. Despite the complexity and mystery of the science of glass, glass technology has continued to advance from sheet glass to crystal glass, optical glass and prisms, to float glasses, chemical glassware, fibre optics and metal glasses.
In Our Time: Chromatography
In Our Time is a wonderful series on BBC Radio 4.
Melvyn Bragg and guests discuss the origins, development and uses of chromatography. In its basic form, it is familiar to generations of schoolchildren who put a spot of ink at the bottom of a strip of paper, dip it in water and then watch the pigments spread upwards, revealing their separate colours. Chemists in the 19th Century started to find new ways to separate mixtures and their work was taken further by Mikhail Tsvet, a Russian-Italian scientist who is often credited with inventing chromatography in 1900. The technique has become so widely used, it is now an integral part of testing the quality of air and water, the levels of drugs in athletes, in forensics and in the preparation of pharmaceuticals.
Melvyn Bragg and guests discuss the origins, development and uses of chromatography. In its basic form, it is familiar to generations of schoolchildren who put a spot of ink at the bottom of a strip of paper, dip it in water and then watch the pigments spread upwards, revealing their separate colours. Chemists in the 19th Century started to find new ways to separate mixtures and their work was taken further by Mikhail Tsvet, a Russian-Italian scientist who is often credited with inventing chromatography in 1900. The technique has become so widely used, it is now an integral part of testing the quality of air and water, the levels of drugs in athletes, in forensics and in the preparation of pharmaceuticals.
Energy Changes and Rates of Reaction
The Science of Fireworks
In addition to being a good demonstration lecture, Professor Bishop also explains the factors that affect the rate of a chemical reaction, with some rather cool demonstrations.
Through demonstrations of pyrotechnic chemistry hear how Chinese incendiaries made from honey led to the development of gunpowder; discover how the loud bangs of fireworks are routed in the origins of photography; and find out how an accident in a nineteenth-century kitchen sparked a new chemistry for firework making.
Through demonstrations of pyrotechnic chemistry hear how Chinese incendiaries made from honey led to the development of gunpowder; discover how the loud bangs of fireworks are routed in the origins of photography; and find out how an accident in a nineteenth-century kitchen sparked a new chemistry for firework making.





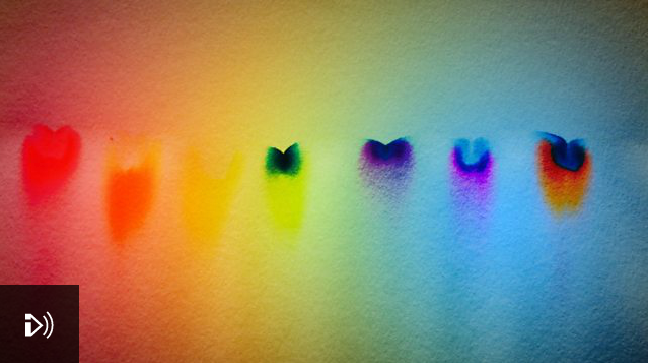

Teaching Science